Exploring antibody validation approaches with AbVal meeting speakers: Thermo Fisher Scientific
5
Min Read
In this blog:
- We speak with Anna Cartier, PhD - Product Manager, Antibodies, from Thermo Fisher Scientific about their antibody validation
- We explore data provided from the validation of their Vimentin Monoclonal Antibody (V9)
- Attend the 4th International Antibody Validation Meeting to hear more from David Piper, Senior Director, R&D, Protein and Cell Analysis
This September, the 4th International Antibody Validation Meeting will be returning to Bath.
We are really excited to be running the meeting once again. This event aims to provide a unique forum to improve and support antibody validation through productive debate and sharing between reagent suppliers, researchers in academia and the biotech/biopharma sector.
In a build up to the discussions and talks taking place at the event, we are taking a deeper look at the validation of antibody suppliers presenting during the conference. Today, we learn about Thermo Fisher Scientific’s validation approaches.
Thermo Fisher Scientific concentrates on tailoring their methodology to the antibody target, sample type and relevant application instead of focusing on a singular method for antibody specificity validation. Some examples of methodologies they use include knockout, knockdown, cellular treatment, and relative expression. These approaches cover a range of applications such as western blotting (WB), immunocytochemistry (ICC), immunohistochemistry (IHC), flow cytometry, and ChIP assays.
For this blog, we are taken through the specific example of validation of the Vimentin Monoclonal Antibody (V9) eBioscience™ (SKU # 14-9897-82*) with corresponding data, outlined to us by Anna Cartier, PhD.
Using multiple approaches to validate antibodies
Selecting the right antibody validation approach
In order to select the appropriate antibody specificity testing method(s), Thermo Fisher Scientific primarily considers two key factors.
- Nature of the target
This involves considering relevant target properties such as biological function, sub-cellular localization, post-translational modifications, gene essentiality, and the rate of turnover.
- Nature of the sample type
Sample type is also considered due to its influence on epitope exposure and the reactivity of an antibody in a specific application.
Complementary validation approaches in practice
To see how this works in practice, we explore the antibody validation approach taken with the Vimentin Monoclonal Antibody (V9) eBioscience™ (SKU # 14-9897-82*).
In this example, the Vimentin Monoclonal antibody was being tested for specificity in western blotting. Genetic modification and relative expression were selected as complementary testing methods based on the target and sample type.
Genetic modification was performed by generating a knockout cell line. As shown in figure 1, the absence of the band of interest corresponding to vimentin signal is seen when compared to control cells. The lack of antibody reactivity gives confidence in its specificity to bind to the correct target.

Alongside this approach, relative expression was used. A panel of lysates from cell lines expressing high, medium, low, and no vimentin was tested. Here, the ability of the antibody to detect variations in vimentin expression levels at the expected molecular weight confirms its specificity in WB, as shown in the figure below.
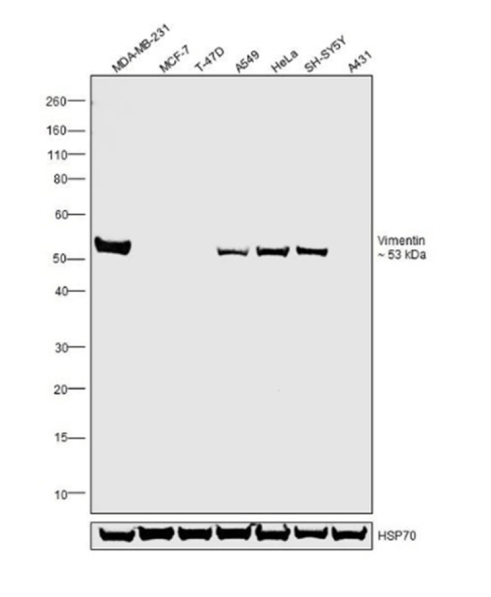
Monoclonal Antibody (V9), eBioscience™ (Product # 14-9897-82*) across cell lines tested
demonstrates antibody specificity by WB.
To further show their tailored method to validation, when testing this antibody’s specificity in ICC the strategy of positive and negative cell models was used. As shown in figure 3, the staining patterns of vimentin were compared and the presence or absence of vimentin detection as well as the correct cellular localization in positive cells helped to demonstrate antibody specificity by ICC.

positive and vimentin-negative cell lines using Anti-Vimentin Monoclonal Antibody (V9),
eBioscience™ (Product # 14-9897-82*) demonstrates antibody specificity by ICC.
*For Research Use Only. Not for use in diagnostic procedures.
–
This example goes to demonstrate how multiple approaches to antibody validation can be used for particular antibodies or applications being tested. By making use of complementary methods that are right for the specific context, researchers can have increased confidence the antibody is specific to the target.
Interested in learning more?
Thermo Fisher Scientific will be attending the 4th International Antibody Validation Meeting this September, where David Piper, PhD will be speaking with a talk titled: ‘Advanced antibody validation: a commitment to antibody performance’
When asked what they were looking forward to at the meeting, they said:
‘Thermo Fisher Scientific is committed to helping further scientific discoveries and seeks to provide customers with high quality reagents to ensure the reproducibility of their work. We continuously expand this rigorous testing throughout our catalog and work to enhance the digital experience, making these products and relevant experimental data easy to find in our digital search experience. We’re excited to share an overview of our efforts at this meeting and get feedback from researchers on what else we can do to support their antibody-based research.’
You can see who else will be speaking at the meeting, browse the full programme, and find out more about the sponsors for the event over on the meeting website.
We are really looking forward to all the productive discussions on validation to come this September!
- Skye and the CiteAb team
